Abstract
[Background]
Reduced-intensity conditioning (RIC) regimens before allogeneic hematopoietic cell transplantation (HCT) have become dramatically more popular worldwide. Many studies have suggested that RIC is associated with reduced nonrelapse mortality (NRM) but increased relapse, which results in survival outcomes comparable to those with myeloablative conditioning (MAC). Although the benefit of RIC regimens for NRM might be increased by baseline characteristics such as age and comorbidities, it is unclear how we should select conditioning intensity. In this Japanese nationwide retrospective study, we propose a novel scoring system to individualize conditioning intensity for patients aged in their 50's or 60's where physicians often face a dilemma in selecting conditioning regimens.
[Methods]
This retrospective study included patients aged 50-69 years with AML or ALL in first or second complete remission, and MDS who underwent their first allogeneic HCT from HLA-matched related donors, HLA-matched unrelated donors, HLA-mismatched unrelated donors, or umbilical cord blood between 2008 and 2019. We identified 6147 patients who fulfilled the eligibility criteria.
The Risk assessment for the Intensity of Conditioning regimen in Elderly patients (RICE) score was developed as follows: First, to generate and validate the RICE score, we randomly divided patients with complete information of all covariates into the training and validation cohort in a 2:1 ratio. Second, in the training cohort, we identified the potential covariates having the interactions with conditioning intensity (MAC vs. RIC) on NRM and included all selected interaction terms in addition to recipient's age at HCT and HCT-CI which are theoretically important interaction terms in the final model. Based on the final model, we assign the weight for the RICE score. Third, we compared MAC to RIC regimens stratified by the RICE scores.
This study was performed in accordance with the Declaration of Helsinki and was approved by the data management committee of JSTCT and by the Institutional Review Board of Jichi Medical University Saitama Medical Center.
[Results]
In the training cohort, 2223 (54.3%) and 1873 (45.7%) received MAC and RIC regimens. The median age at HCT was 57 (range, 50 to 69 years) and 61 years (range, 50 to 69 years) in MAC and RIC (P < 0.001). The most common regimen in MAC and RIC consisted of Fludarabine/Busulfan-based conditioning. We determined the interaction between conditioning intensity and each covariate in the training cohort. Recipient's age at HCT, HCT-CI, and UCB were used to design a final model to predict the difference in an individual patient's risk of NRM between MAC and RIC - the RICE score. The RICE score is calculated by summing the number of factors present at HCT (Age [≥ 60], HCT-CI [≥ 2], and UCB). Patients were then assigned to two groups on the basis of their number of factors: 0-1, the low RICE score; or 2-3, the high RICE score.
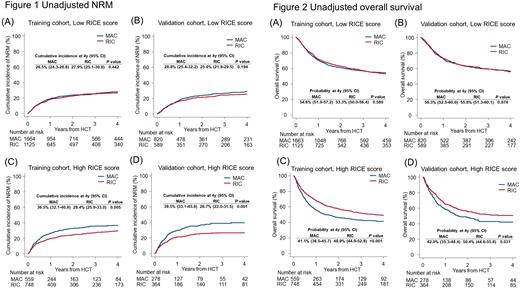
In the multivariate analyses, we found no significant differences on NRM between MAC and RIC in patients with the low RICE score (training cohort: HR, 0.99, 95%CI, 0.85-1.15, P = 0.860; validation cohort: HR, 0.81, 95%CI, 0.66-1.01, P = 0.061) (Figure 1A and 1B). In contrast, RIC was significantly associated with a decreased risk of NRM in patients with the high RICE score (training cohort: HR, 0.73, 95%CI, 0.60-0.90, P = 0.003; validation cohort: HR, 0.57, 95%CI, 0.43-0.77, P < 0.001) (Figure 1C and 1D). There were no significant differences in relapse between the MAC and RIC groups regardless of the RICE score. We also observed the significant difference on overall survival between MAC and RIC only in patients with the high RICE score (Figure 2A-D).
Finally, the training cohort and validation cohort were combined to validate the robustness of the RICE score. In the multivariate analysis limited to either AML (n = 2818), ALL (n = 1140), MDS (n = 2189), or Fludarabine/Busulfan-based regimen (n = 3183), RIC was significantly associated with a lower risk of NRM compared to MAC in patients with the high RICE score, but we observed no difference between MAC and RIC in patients with the low RICE score.
[Conclusion]
We have developed the RICE score that could identify the populations who have significant benefits on NRM if they underwent HCT with RIC. This simple and validated interaction analysis-based scoring system would help us to choose appropriate conditioning regimens and improve transplant outcomes.
Disclosures
Sawa:GSK plc.: Honoraria; Daiichi Sankyo Co., Ltd.: Honoraria; AstraZeneca K.K.: Honoraria; Janssen Pharmaceutical K.K.: Honoraria; SymBio Pharmaceuticals Ltd.: Honoraria; CSL Behring K.K.: Honoraria; AbbVie G.K.: Honoraria; Mundipharma K.K.: Honoraria; Shire plc: Honoraria; Mochida Pharmaceutical Co., Ltd.: Honoraria; Celgene K.K.: Honoraria; Sanofi K.K.: Honoraria; Takeda Pharmaceutical Co., Ltd.: Honoraria; Sumitomo Dainippon Pharma Co., Ltd.: Honoraria; Otsuka Pharmaceutical Co., Ltd.: Honoraria; Eisai Co., Ltd.: Honoraria; Novartis Pharma K.K.: Honoraria; Asahi Kasei Pharma Corp.: Honoraria; Bristol-Myers Squibb K.K.: Honoraria; MSD K.K.: Honoraria; Ono Pharmaceutical Co., Ltd.: Honoraria; Nippon Shinyaku Co., Ltd.: Honoraria; Astellas Pharma Inc.: Honoraria; Pfizer Japan Inc.: Honoraria; Chugai Pharmaceutical Co., Ltd.: Honoraria; Kyowa Kirin Co., Ltd.: Honoraria. Kanda:asclepia: Honoraria; ASAHI KASEI PHARMA CORPORATION: Honoraria; Janssen Pharmaceutical K.K.: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb Co: Honoraria; Novartis Pharma K.K.: Honoraria, Membership on an entity's Board of Directors or advisory committees; TEIJIN PHARMA LIMITED.: Honoraria; CHUGAI PHARMACEUTICAL Co., Ltd.: Honoraria; Takeda Pharmaceutical Company Limited: Honoraria; Sumitomo Dainippon Pharma Co., Ltd.: Honoraria; DAIICHI SANKYO Co., Ltd.: Honoraria, Membership on an entity's Board of Directors or advisory committees; SymBio Pharmaceuticals, Ltd.: Membership on an entity's Board of Directors or advisory committees; Sanofi K.K.: Honoraria; Kyowa Kirin Co., Ltd.: Honoraria; Megakaryon Co: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen Pharmaceutical K.K.: Honoraria; Ono Pharma Inc.: Honoraria; Otsuka Pharmaceutical Co., Ltd.: Honoraria; Amgen Pharma Inc.: Honoraria; AbbVie Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas Pharma Inc.: Consultancy, Honoraria; MSD K.K.: Honoraria; CSL Behring K.K.: Honoraria; Otsuka Pharmaceutical Co., Ltd.: Honoraria; NIPPON KAYAKU CO.,LTD.: Honoraria; Nippon Shinyaku Co., Ltd.: Honoraria; Eisai: Research Funding. Kanda:Mundipharma Pharmaceuticals: Research Funding. Atsuta:Mochida Pharmaceutical Co., Ltd.: Honoraria; Meiji Seika Pharma Co, Ltd.: Honoraria; Kyowa Kirin Co., Ltd: Honoraria; AbbVie GK: Honoraria; Novartis Pharma KK: Honoraria; Astellas Pharma Inc.: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal